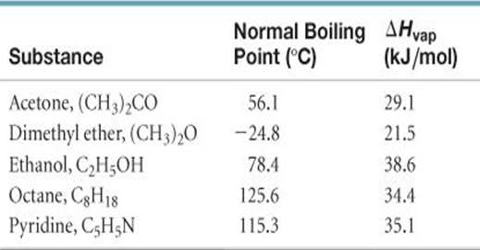
Acetone heat capacities: (c L and c G ) for the molecular liquid and... | Download Scientific Diagram

OneClass: 2) The normal boiling point of acetone is 56.5°C At an elevation of 5300 ft, the atmospher...
How to calculate the vapor pressure of acetone at 25.0°C if the enthalpy of vaporization for acetone is 32.0 kJ/mol and the normal boiling point of acetone is 56.5°C - Quora
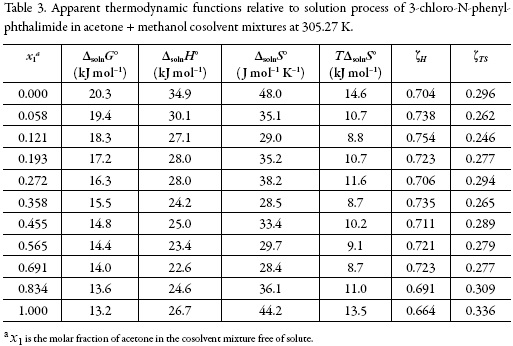
Solution thermodynamics and preferential solvation of 3-chloro-N-phenyl-phthalimide in acetone + methanol mixtures
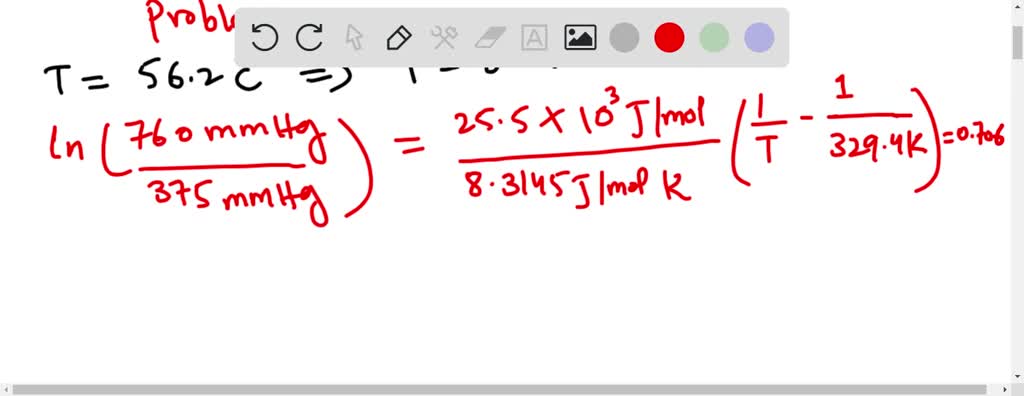
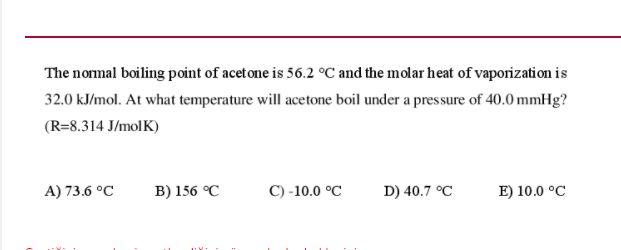
SOLVED:The normal boiling point of acetone is 56.2^{\circ} \mathrm{C}, and the molar heat of vaporization is 32.0 \mathrm{kJ} \mathrm{mol}^{-1}. What is the boiling temperature of acetone under a pressure of 50.0 \mathrm{mmHg} ?

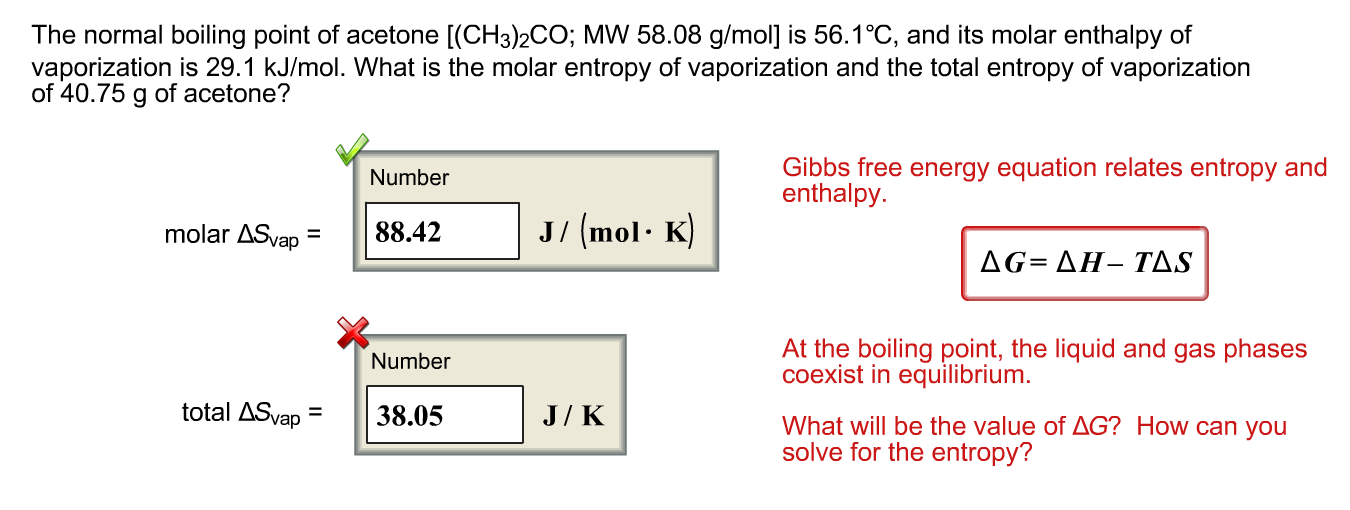
The heat of vaporization of acetone, CH_3COCH_3, at its boiling point is 29.1 kJ/mol. How much energy is required to vaporize 125.0 g of acetone at its boiling point? | Study.com

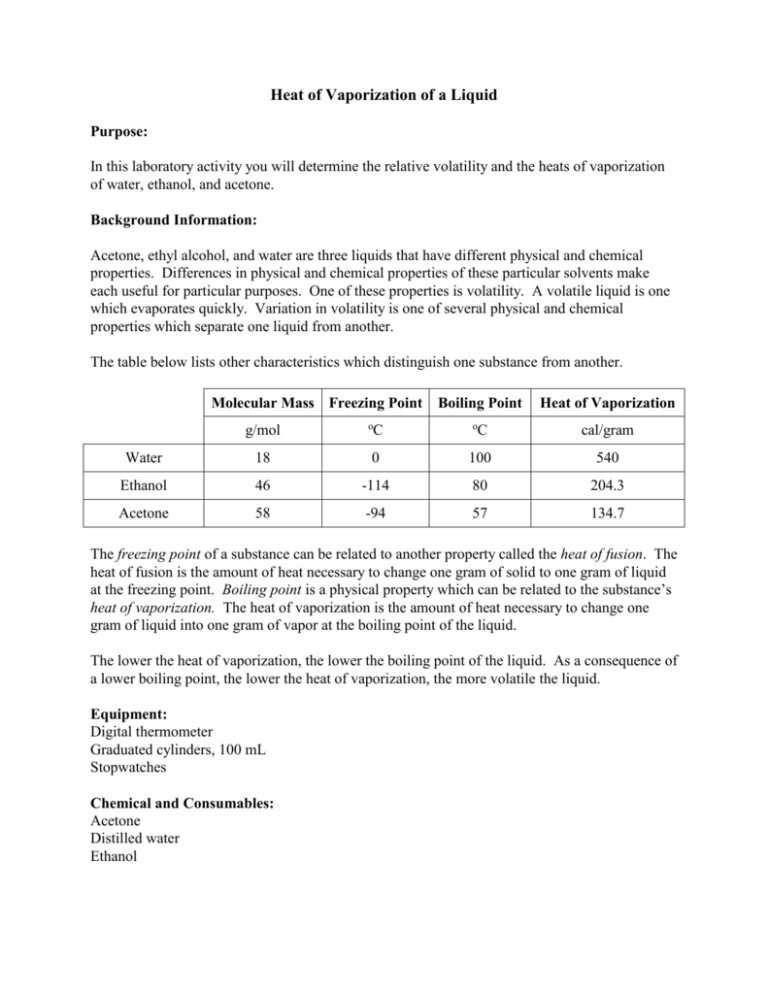
SOLVED:Equatlon and Table To solve this problem; You will need use the Clausius-Clapeyron equation 4Hyep and this table of boiling points and heat = vaponzation for liquids Liquid Formula Normal boiling Heat

A theoretical analysis on enthalpy of vaporization: Temperature-dependence and singularity at the critical state - ScienceDirect

OneClass: A standard entropy of vaporization of acetone is approximately 85jk/mol at its boiling poin...
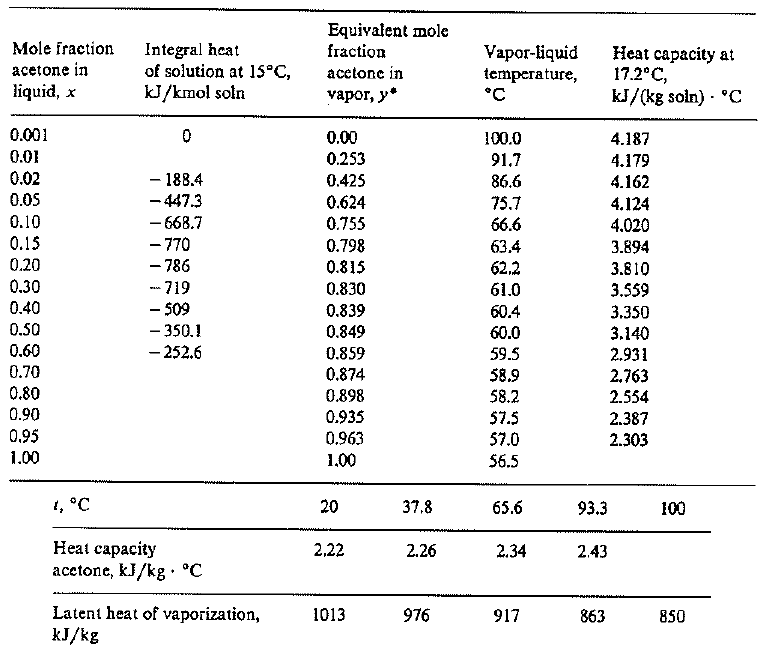
![PDF] Heat Capacities of Aqueous Solutions of Acetone; 2,5-Hexanedione; Diethyl Ether; 1,2-Dimethoxyethane; Benzyl Alcohol; and Cyclohexanol at Temperatures to 523 K | Semantic Scholar PDF] Heat Capacities of Aqueous Solutions of Acetone; 2,5-Hexanedione; Diethyl Ether; 1,2-Dimethoxyethane; Benzyl Alcohol; and Cyclohexanol at Temperatures to 523 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/87d097be65b5ce058a11c2e59323aff45a229c41/6-Table1-1.png)
PDF] Heat Capacities of Aqueous Solutions of Acetone; 2,5-Hexanedione; Diethyl Ether; 1,2-Dimethoxyethane; Benzyl Alcohol; and Cyclohexanol at Temperatures to 523 K | Semantic Scholar

`Delta_(vap)`S1 of acetone is `93.0 JK^(-1) "mol"^(-1)`.If boiling point of acetone is `56^(@)C`, calculate the heat required for the vaporisation of 1 g of acetone.
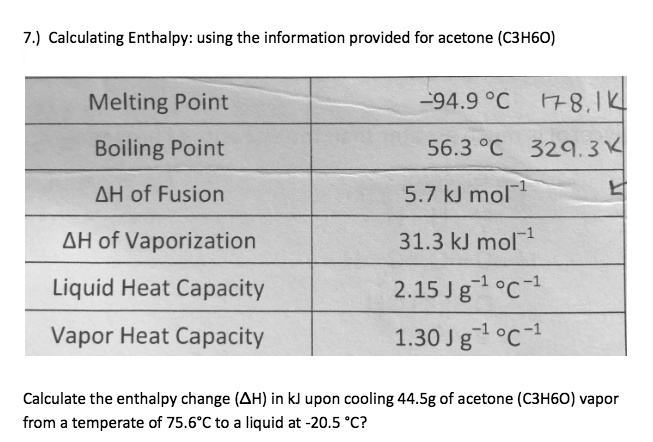
SOLVED:7.) Calculating Enthalpy: using the information provided for acetone (C3HGO) Melting Point Boiling Point AH of Fusion ~94.9 %€ +7-8,1444 56.3 "C 329.34 5.7 kJ mol-1 AH of Vaporization 31.3 kJ mol-1