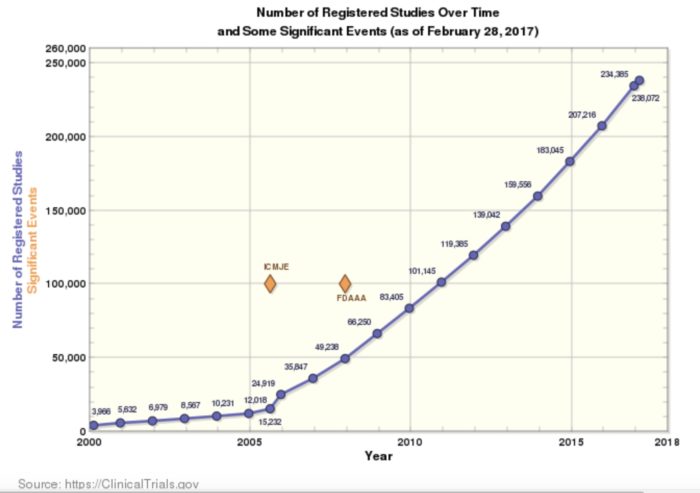
Celebrating 20 Years of ClinicalTrials.gov and Looking to the Future – NLM Musings from the Mezzanine

Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis | The BMJ

Celebrating 20 Years of ClinicalTrials.gov and Looking to the Future – NLM Musings from the Mezzanine

Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet

Celebrating 20 Years of ClinicalTrials.gov and Looking to the Future – NLM Musings from the Mezzanine

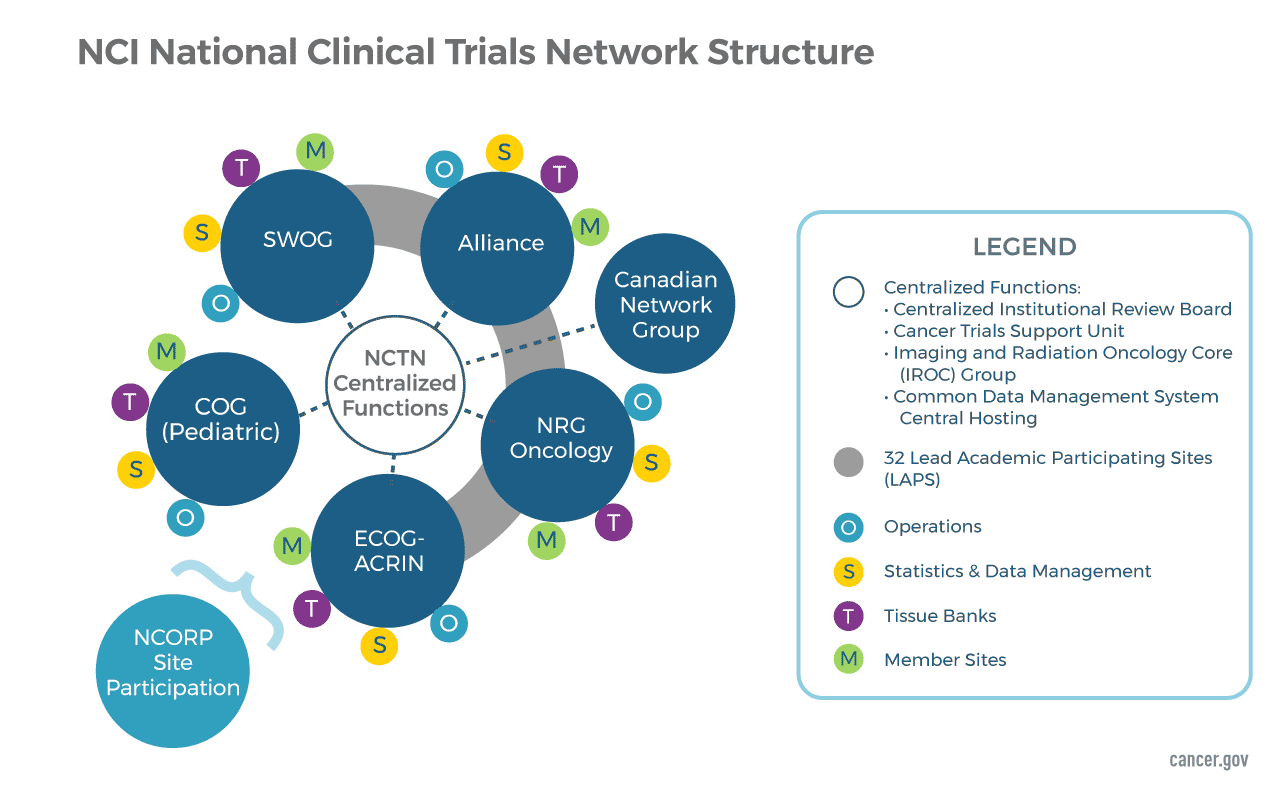


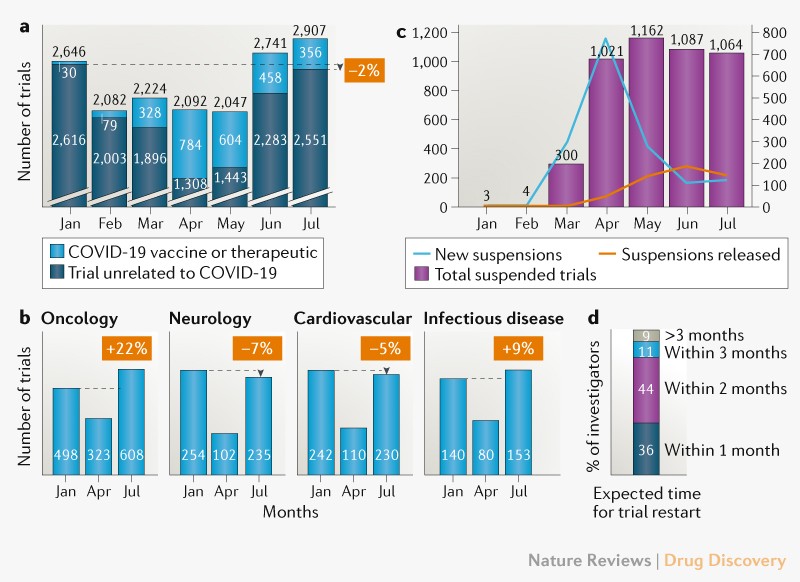


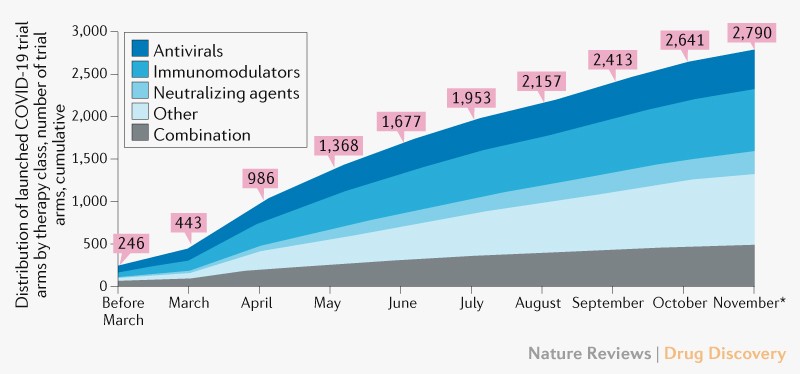








![Withdrawn] Clinical Trials Regulation - GOV.UK Withdrawn] Clinical Trials Regulation - GOV.UK](https://assets.publishing.service.gov.uk/government/uploads/system/uploads/image_data/file/77311/s960_Digital_logo-for-gov-uk_-_Copy.png)