Radiation Therapy Digital Data Submission Process for National Clinical Trials Network - International Journal of Radiation Oncology, Biology, Physics

ClinicalTrials.gov trial registration submission cycle. JHUSOM: Johns... | Download Scientific Diagram

Clinical Submission Process Guide - OptumHealth … / clinical-submission-process-guide-optumhealth.pdf / PDF4PRO

New industry position requires submission for journal publication of all phase III clinical trials - IFPMA

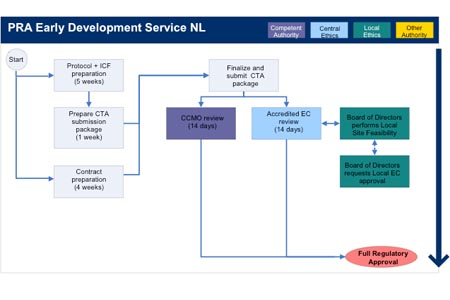
Standards in the operational management of clinical trials and their... | Download Scientific Diagram

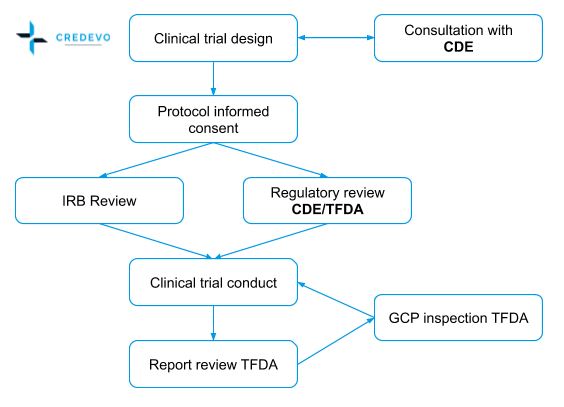
An overview of the procedure for clinical trial applications and the... | Download Scientific Diagram

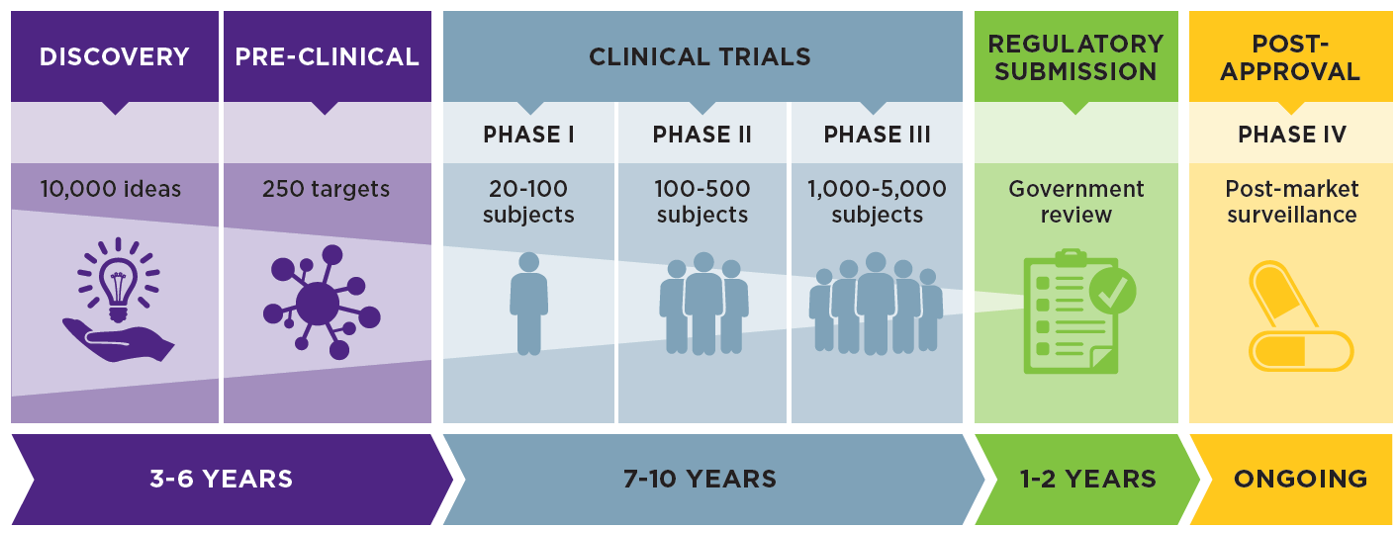
Key steps in the National Cancer Institute (NCI) clinical trial review... | Download Scientific Diagram
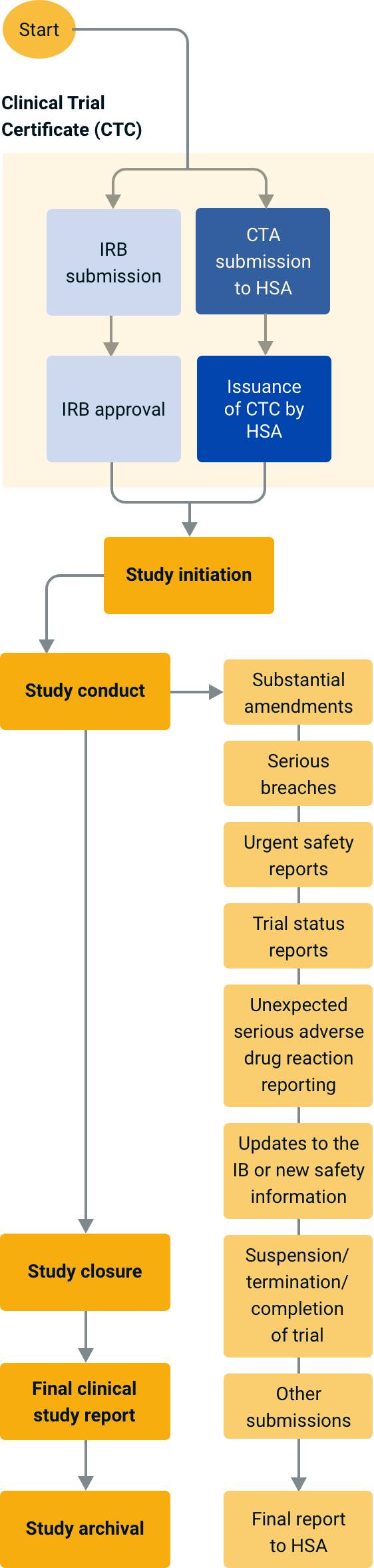
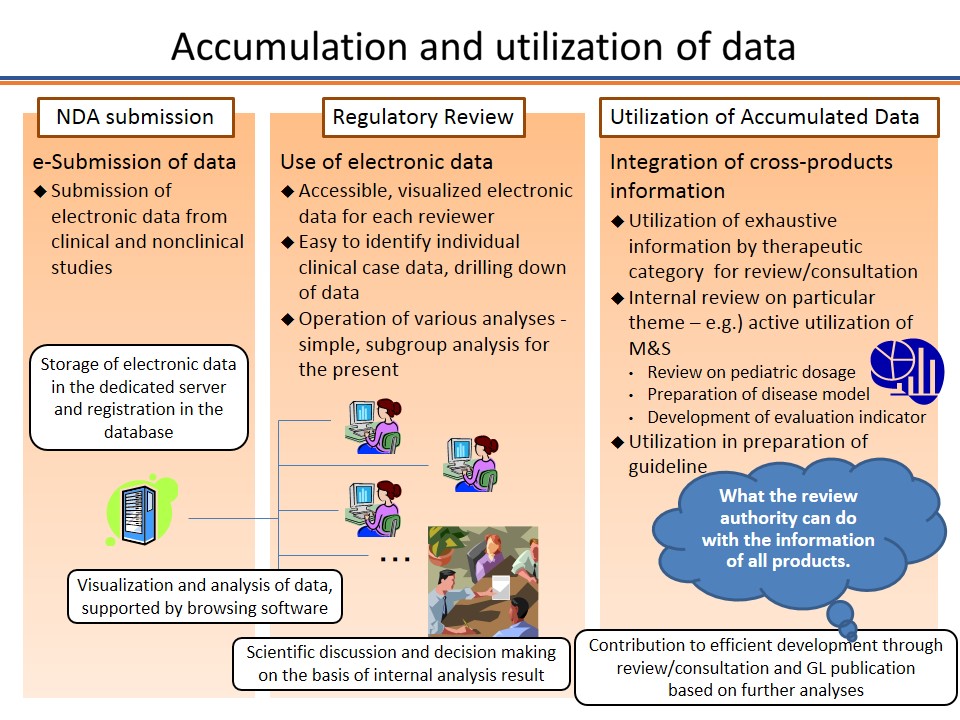
Lessons Learned for Successful e-Study Data Submission to PMDA toward the End of Transitional Period