SARQA/DKG Conference 3-4 October SARQA/DKG Conference 3-4 OCTOBER 2002 Annex 13 Update An Industry Perspective Michael J Cooke Director, Global. - ppt download
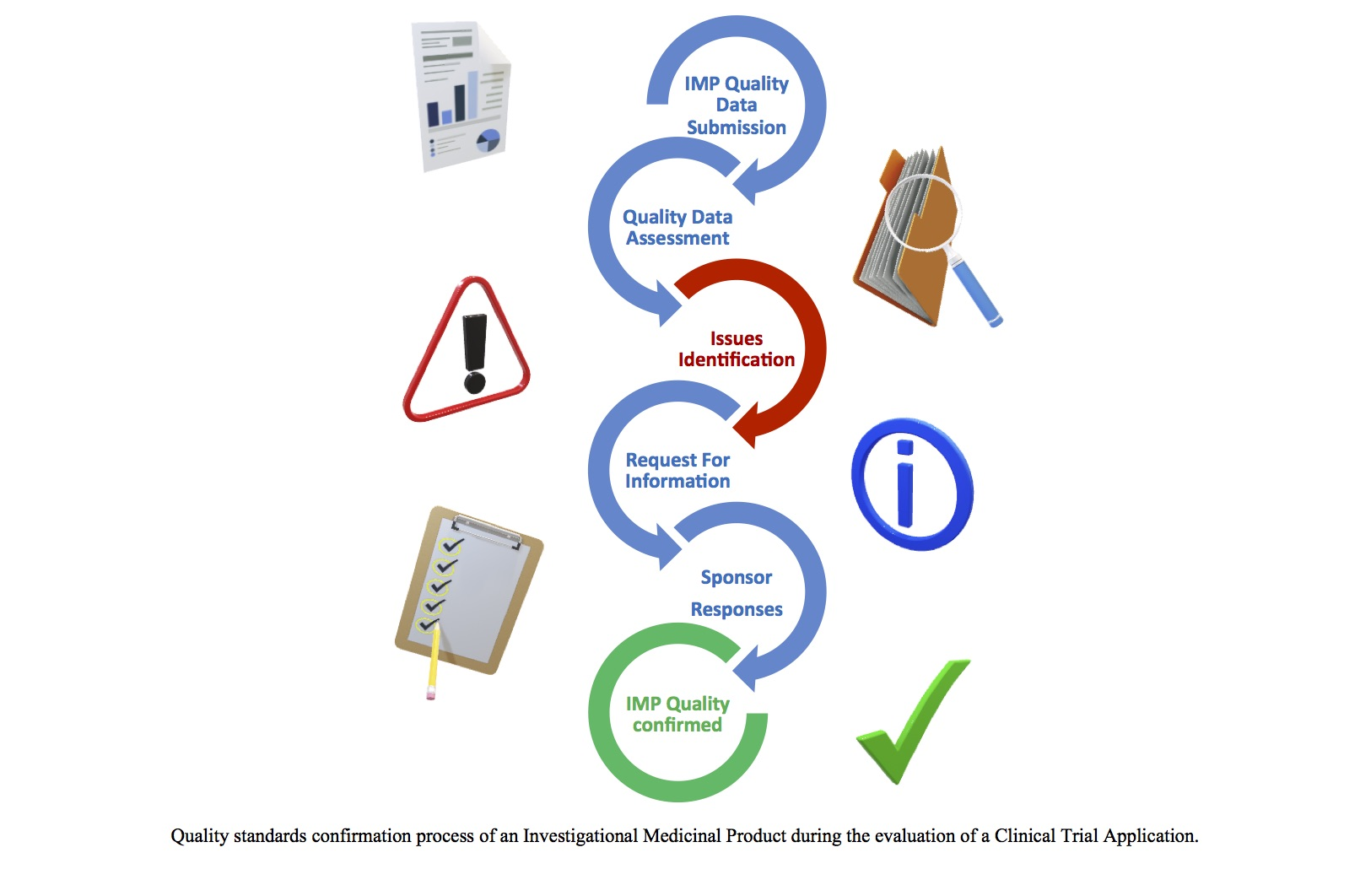
Pharmaceuticals | Free Full-Text | Quality Assessment of Investigational Medicinal Products in COVID-19 Clinical Trials: One Year of Activity at the Clinical Trials Office | HTML

Eu Gmp Annex 13 (Investigational Medicinal Products , Complexity Meets Reality) Songs Download - Free Online Songs @ JioSaavn

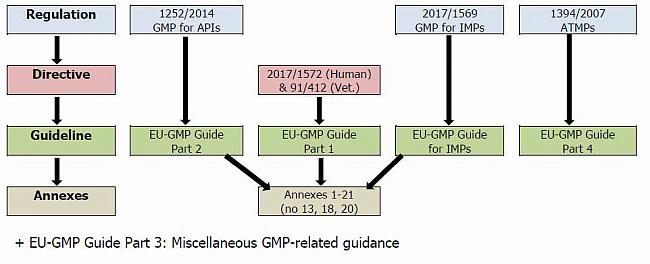
Guidance Document - Annex 13 to the Current Edition of the Good Manufacturing Practices Guidelines - Orion GMP Solutions

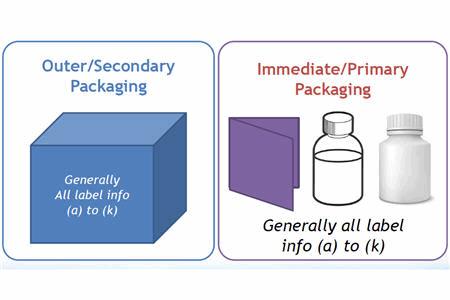
Solve Expiry Labels, DtP, and Timelines for EU 536/2014 Clinical Trials Regulation | Healthcare Packaging